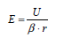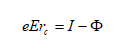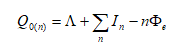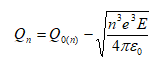Field Ion Microscopy (FIM)
In 1928 Fowler and Nordheim [Fowler, 1928] published their theoretical work about the emission of electrons from solids triggered by an external electrical field. The necessary field strength of about 10 V/nm didn't seem to be manageable technically. But in 1935 Erwin Müller used needle shaped tips. The enhanced electrical field strength at the apex was sufficient to realize the necessary conditions for field emission. Müller constructed the Field Emission Microscope (FEM), an instrument consisting of a detector, a phosphorous screen, and the needle shaped tips within an evacuated glass cylinder. By applying a negative voltage to the tip, electrons are emitted, creating a high resolution image of the tip surface.
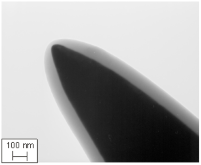
Although high resolution images became possible, atomic resolution was not achievable. The wave length of the electron limited the FEM. The situation changed if a positive voltage was applied to the tip. In this case, Müller observed the desorption of tip atoms. The resulting images were faint, because the amount of ionized atoms was limited, while electrons in the former instrument were unlimited. To increase the image contrast, an imaging gas was inserted into the vacuum cylinder. The gas was supposed to deliver a reasonable amount of ionizable atoms. This instrument was named Field Ion Microscope (FIM), delivering the first images of single atoms in 1952 [Müller, 1952]. Both instruments, the FIM and the FEM, make use of needle shaped specimens to achieve the necessary field strengths with moderate high voltage sources. Modern 3D atom probe (3DAP) instruments still use the same specimen shape. The typical radius of curvature of these specimens is in the range of 30 to 50 nm. The field strength E is given by
if a voltage U is applied to a tip of curvature radius r. The field compression factor t β is typically in the range of 2 to 12. It is an instrument and tip specific parameter. The typical range of values for the used instruments amounts to 4 to 9.
In principle, the FIM consists of a sample tip in front of a detector setup mounted inside a vacuum chamber. Modern vacuum chambers are made of stainless steel due to improved processing techniques which have not been available in 1952.
An inert imaging gas - typically neon (Ne) or helium (He) - is inserted into the chamber with a partial pressure of 1*10-5 mbar.
Two processes for image formation have to be distinguished, the ionization of gas atoms and the evaporation of tip atoms. The ionization of gas atoms is the main process for image formation in the FIM
Field Ionization
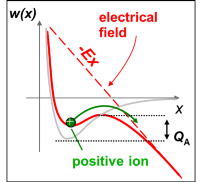
Close to the tip uncharged atoms of the image gas are polarized by the inhomogeneous field and accelerated towards the tip. The atoms collide with the tip surface, loose a part of their kinetic energy and are reflected. This process is repeated several times until the kinetic energy is reduced to a few meV. Finally, the gas atoms will be trapped in regions of high field strength close to the tip. Since the surface is not flat on an atomic scale, the protruding atoms represent regions of highest field strength. Gas atoms will be predominantly adsorbed at these points but cannot be ionized directly. The reason for this becomes obvious by studying figure 2‑4 the following figure ( a) to c) ). To ionize an atom an electron has to be removed. This electron is trapped within a symmetrical potential well if no external field is present. In order to remove the electron, the ionization energy I has to be summonned up. In the presence of an inhomogeneous electrical field the potential well is deformed in an asymmetrical way. Close to a metal surface a finite barrier is formed which can be penetrated with a given tunneling probability, but only if an adequate unoccupied energy level is available in the solid. If no unoccupied energy level is available at a given energy, tunneling is forbidden by the Pauli principle (Figure 2‑4 c figure c)). Only in a given distance - the critical distance - the energy of the electron is above the Fermi energy of the solid and the tunneling probability is still sufficient to allow the penetration of the barrier. This is illustrated in Figure 2‑4 b figure b. Atoms adsorbed at the surface cannot be ionized and get trapped. They increase the field strength even more and provide the necessary distance to ionize atoms in a second layer.
Here, excitations from photon interactions are neglected. For more details please refer to [Miller2000]. From experimental data, it became obvious that the total energy of the gas ions was slightly less than the energy expected, if the ions would originate directly from the metal surface.
Φ = work function
I = ionization energy
Obviously the origin of the ions is therefore a few tenths of nanometers above the metal surface. It is a narrow zone of 0.015 to 0.025 nm in extension/dimension. Due to the small dimensions of this ionization sphere it is very unlikely that incoming gas atoms - before they have lost most of their kinetic energy - will become ionized directly. The tunneling probability is proportional to the number of possible attempts and thus to the retention time of the gas atom within the zone. Incoming gas atoms as well as reflected ones cross the zone very fast, reducing the ionization probability. Adsorption is therefore a necessary process step.
Field evaporation
If the voltage is increased even further also atoms of the specimen itself are ionized. This process is called field evaporation. The process of field evaporation is rather complex and of quantum mechanical nature. Till today no general accepted theory exists.
The specimen surface atoms will be uncharged if no electric field is present. To evaporate an atom out of the lattice, the ionization energy I and binding energy Λ have to be summonned up. But the work function Φ e is gained back when the electrons return to the surface.
If an external field is present, situation changes as demonstrated in Figure 2‑5. The ionic state becomes more stable than the atomic state at distances r away from the metal surface. Thus desorption of an ionized surface atom becomes possible if the reduced energy barrier is overcome by thermal activation.
The rate of evaporation N is proportional to
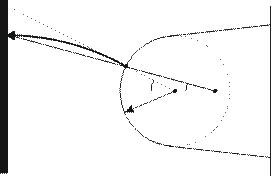
The ionized atoms are accelerated towards the detector system and gain over 90% of their kinetic energy within the first μm. Owing to the inhomogeneity of the electrical field the trajectories of the ions can be dealt to a good approximation as straight lines with instantaneous acceleration at the beginning. The ionized atoms produce a magnified image of the specimen surface. The magnification M is determined by the distance L between tip and detector and the radius R of curvature which is a function of the applied voltage.
The image is a projection of the curved tip surface onto the 2D detector. It is isogonal with regard to the crystallographic axis. The image forming process can be described by /as a point projection.
Specimens are cooled down to cryogenic temperatures to increase the resolution of the produced images. Vibrations of atoms around their rest position lead to aberrations in trajectories and thus to a blurred image.